
First things, first. You need to understand that for a reaction to happen the particles need to have enough energy so that when they collide their bonds will break. However when their bonds reform energy is given out.
- So now it is obvious to note that if the energy REQUIRED to break the bonds is greater than the energy given out when bonds are formed then the temperature of the surroundings will drop. This is an ENDOTHERMIC reaction. ENDO meaning energy taken in.
- When the energy RELEASED when bonds form is greater than energy taken in to break the bonds then the temperature of the surroundings increases. This is an EXOTHERMIC reaction. EXO meaning given out.
Now that that is clear you might begin to wonder about combustion and respiration. They both release energy, do they not? They are indeed exothermic reactions, where a compound is oxidised (reacted with oxygen).
As an exmple of endothermic reaction, we have thermal decomposition of carbonates or even melting (you require heat to melt ice - it is a reaction).
ENTHALPY PROFILE DIAGRAMS:

When one of these crosses your path do not turn the other way and run - bad idea, never turn your back on your enemy. These are free marks in the exam. Think about what happens in EXO and ENDOthermic reactions.
We know that in EXOthermic reaction energy is give out, which means that the products have less energy than reactants. Simple.
In ENDOthermic reactions energy is taken in so the products will have more energy than the reactants.
What's the little hills, you ask?? Remember when I had mentioned that bonds need to be broken first? This is the MINIMUM energy required to do this.
ACTIVATION ENERGY: Minimum energy required to break bonds to start a reaction.
STANDARD CONDITION: A pressure of 100kPa (1 atmosphere) and a stated temperature (25 degrees Celsius).
ENTHALPY CHANGE OF REACTION: Enthalpy change that accompanies a reaction in the molar quantities expressed by chemical equation under standard conditions at standard state.
ENTHALPY CHANGE OF FORMATION: The enthalpy change that takes place when one mole of a substance is formed from it's constituent elements, under standard conditions at standard states.
ENTHALPY CHANGE OF COMBUSTION: The enthalpy change that takes place when one mole of a substance reacts completely with oxygen under standard conditions, at standard states.
Sorry, you just need to memorise them. Try saying them in reverse (not the letters in a word but the whole sentence) and in different order, it helps.
When you are given experimental values you have to use this equation to calculate the enthalpy change.

Pretty self explanatory.
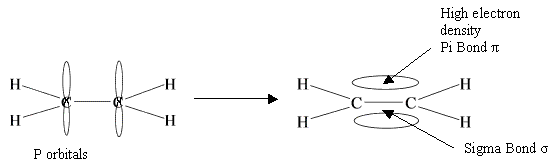
BOND ENTHALPY: The enthalpy change that takes place when one mole of bonds is broken by homolytic fission in gaseous state.
This will always be positive as you are breaking bonds so energy is taken in. When working these out the total bond enthalpy of the reactants equals the bond enthalpy of the products. So when working out the enthalpy change you should do the following:
Enthalpy change = Total bond enthalpy - Total bond enthalpy
of braking bonds of making bonds
In short?? Broken - Made
Hess's law - that old guy - If a reaction can take place by more than one route and the initial and final conditions are the same then the enthalpy change for each route is the same.
Here is how it all works. So try to remember the rules and understand the process. First identify if you're given formation or combustion values. Then you'll know how to work the numbers using the rules.
That's it folks.
This will always be positive as you are breaking bonds so energy is taken in. When working these out the total bond enthalpy of the reactants equals the bond enthalpy of the products. So when working out the enthalpy change you should do the following:
Enthalpy change = Total bond enthalpy - Total bond enthalpy
of braking bonds of making bonds
In short?? Broken - Made
Hess's law - that old guy - If a reaction can take place by more than one route and the initial and final conditions are the same then the enthalpy change for each route is the same.
Here is how it all works. So try to remember the rules and understand the process. First identify if you're given formation or combustion values. Then you'll know how to work the numbers using the rules.
That's it folks.












.jpg)


