Now on to Alkanes brother ALKENE! No excitement? How about this:

Anybody hugging the screen yet? Geez, you people are strange.
As I'm sure you're all aware, because you're smart you all, is that alkenes contain a double bond. This means that they are unsaturated. A double bond means there are TWO pairs of electrons.
First pair forms a sigma bond (that is an overlap of s orbitals - remember those bad boys?). each carbon donates one electron to the SIGMA bond.
Second pair of electrons forms a PI bond (this is sideways overlap of p orbitals on each carbon).
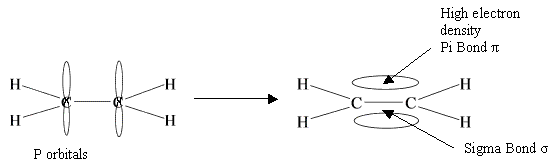

The PI bond fixes the carbon atoms in position, at either end of the double bond. This prevents any rotation of the bond.
Now when we think about the number of bonded regions, we have two on each carbon that is the bond C-H and One C-C bond (even though there's two bonds, it's one region of bonding). This means that the angle is 120 degrees, forming a trigonal planar shape.
Oh, and another thing. The PI bond is a very small region of space, this means there will be a high electron density (almost like pressure). This means that PI bond is weaker as electrophiles will attack that region first.
Oh, and another thing. The PI bond is a very small region of space, this means there will be a high electron density (almost like pressure). This means that PI bond is weaker as electrophiles will attack that region first.
Next up we have reactions of alkenes. There's many so be prepared to take notes.
Addition of HYDROGEN / hydrogenation: 150 degrees Celsius
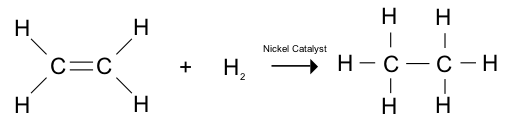

Addition of Hydrogen halides:

Make sure that you know when what fission takes place. Keep in mind that when both elements have the same electronegativity, it will be HOMOLYTIC fission. Different elements always have a difference of electronegativity so it will be a HETEROLYTIC fission.
Electrophile: Electron pair acceptor (positive ion).
There are other things for which alkenes are used for. That is addition polymerisation. It is very simple: monomers of an alkene are added together. During polymerisation the double bond is broken to form a long chain made up of the subunits that were used in the reaction. Ensure you remember that the double bond breaks and draw brackets around that subunit to show it is only a section of the polymer.
Continuing on the topic of polymers is a section of waste. Remember that recycling is important to conserve raw resources, as well as reduce CO2 emission. This needs to be done carefully as some plastics contain Chlorine, which has an effect on ozone layer (about that later). Furthermore, chlorine is toxic and can form HCl, which we know is very corrosive.
To reduce waste biodegradable and decomposable plastics are developed.
Electrophile: Electron pair acceptor (positive ion).
There are other things for which alkenes are used for. That is addition polymerisation. It is very simple: monomers of an alkene are added together. During polymerisation the double bond is broken to form a long chain made up of the subunits that were used in the reaction. Ensure you remember that the double bond breaks and draw brackets around that subunit to show it is only a section of the polymer.
Continuing on the topic of polymers is a section of waste. Remember that recycling is important to conserve raw resources, as well as reduce CO2 emission. This needs to be done carefully as some plastics contain Chlorine, which has an effect on ozone layer (about that later). Furthermore, chlorine is toxic and can form HCl, which we know is very corrosive.
To reduce waste biodegradable and decomposable plastics are developed.


No comments:
Post a Comment