Don't worry this part of the course isn't about actual moles:
Though I'm sure I'll find someone here who will go: Awww, why not ^_^ (Sorry)
When talking about a mole of a substance you can't think about furry little atoms that dig underground all their life, it doesn't work like that, again sorry ^_^
A MOLE of a substance can be explained in many different ways.
- Amount of substance
- The Avogardo's constant (sound cool, doesn't it - you'll learn to love and hate it soon)
If we take IRON with molecular mass of 55.8, for example, the amount of 1 mole of iron would be 55.8 grams. See the connection yet??
Okay, 1 mole of Xenon (why not??) with molecular mass of 131.3 is 131.3 grams. Well... Isn't this magic? Its not, sorry so many disappointments.
Now you may ask if we have 3.5 moles of something how much is that? Well, you might have figured that out already that you'd multiply the molecular mass by 3.5. To make life easier this is the formula:


Okay, 1 mole of Xenon (why not??) with molecular mass of 131.3 is 131.3 grams. Well... Isn't this magic? Its not, sorry so many disappointments.
Now you may ask if we have 3.5 moles of something how much is that? Well, you might have figured that out already that you'd multiply the molecular mass by 3.5. To make life easier this is the formula:
Now, when talking about Avogadro's constant life gets so much more interesting. A mole of ANY substance contains exactly 6.02 × 10^23 mol-1 particles.
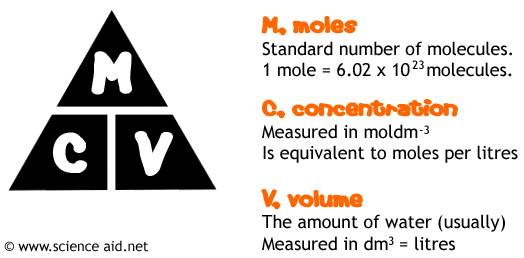
You may also need to calculate concentrations and volume of aqueous solutions so here is another triangle, which is pretty much self explanatory - find what you need to work out, see what you've got,, find the right triangle and then your best friend calculator will do the rest.
Examiners also like to put another type of calculation just to throw you, so watch your units!! You may have to calculate the gas volume of, well, a gas.
Remember that 1 mole of gas molecules will occupy 24 dm^3 at room temperature and pressure.
Well, that's pretty much it at the moment, I mean when it comes to moles it does. Oh, I would have forgotten completely (my chemistry teacher would kill me if I didn't say this).
REMEMBER ABOUT STOICHIOMETRY.
Sounds gibberish? Well all it means is always look at the ratio of moles in the equation you'll be given.
Now for those Linkin Park fans out there: In the end it doesn't even matter cos you know it all, so when they come for you with those equations you'll not be Powerless any more.
No comments:
Post a Comment