
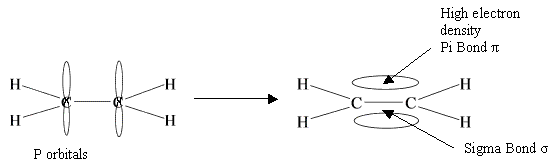
All molecules absorb infrared radiation. This energy that they absorb causes the bond to oscillate in a certain way. It could either be stretching back and forth or a bending of the bonds of to the side.
This absorption shows up on infrared spectrum, and enables us to identify the presence of functional groups. Cool, eh??
You will be given a table in the exam which gives you absorption data and identifies the responsible functional group. Handy. Learn to understand it and identify what's where.

Learn it, learn it and once again learn it! Not of by heart of course but make sure you know what you see when you do see it.
Infrared spectroscopy is very useful and is now used in breathalysers to measure how much alcohol is in your breath.
Next we have mass spectrometry.
It is dead easy. An electron gun fires at a molecules and breaks it up. Just like glass shattering different fragments are formed, big and small. These show up as peaks on the graph. However, unlike glass, the fragments are cations (positive ions), which get acted on by a huge magnet. The further they get pushed away the smaller their mass was (if a person gets hit by a truck, they go flying but if the truck hits a tree - it'll move but slightly). How far they get pushed enables scientists to determine their mass.
Now the peak furthest to the right (biggest m/z value) is the molecular ion. This bad boy is pretty lucky as the gun managed to hit one of the inner electrons and hence the molecule didn't break up at all. It is the molecule you were testing for but charged. Simple??
Once you have the mass you play around with numbers and figure out what it is.

Hey! No sad faces... Not on my watch.
Any questions? Ask, I don't bite... Much.








.jpg)










